of charge e and mass m, are accelerated across the selected high voltage V; energy conservation yields eV = (1=2)mv2, or e=m = v2=(2V), where v is the resulting speed of the electrons in the beam. Since we are using this experiment to measure the ratio e=m, the variables e, m, and v are each unknown.. This chemistry and physics video tutorial provides a basic introduction into the cathode ray tube experiment. JJ Thompson used this experiment to conclude t.
.PNG)
Atoms, molecules and ions Presentation Chemistry
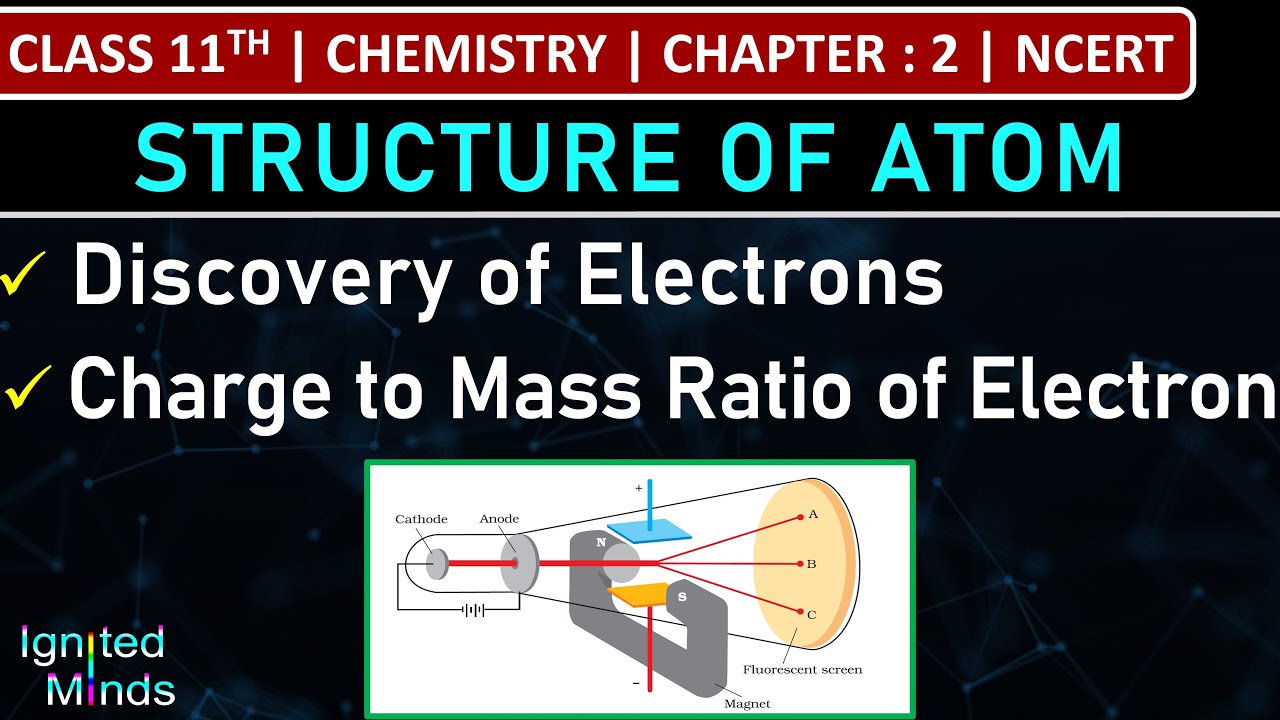
Class 11th Chemistry Discovery of Electrons Charge to Mass Ratio of Electron Chapter 2

Charge to Mass ratio for Electrons YouTube
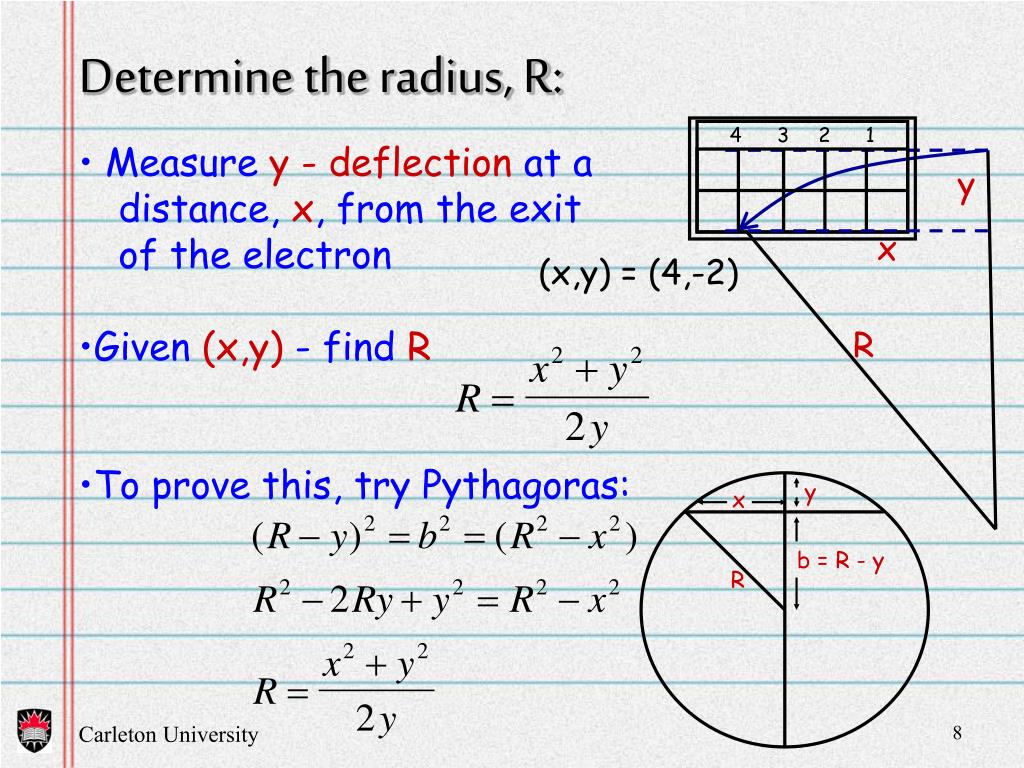
PPT Measurement of Charge to Mass Ratio For an Electron ( Thompson’s Experiment ) PowerPoint
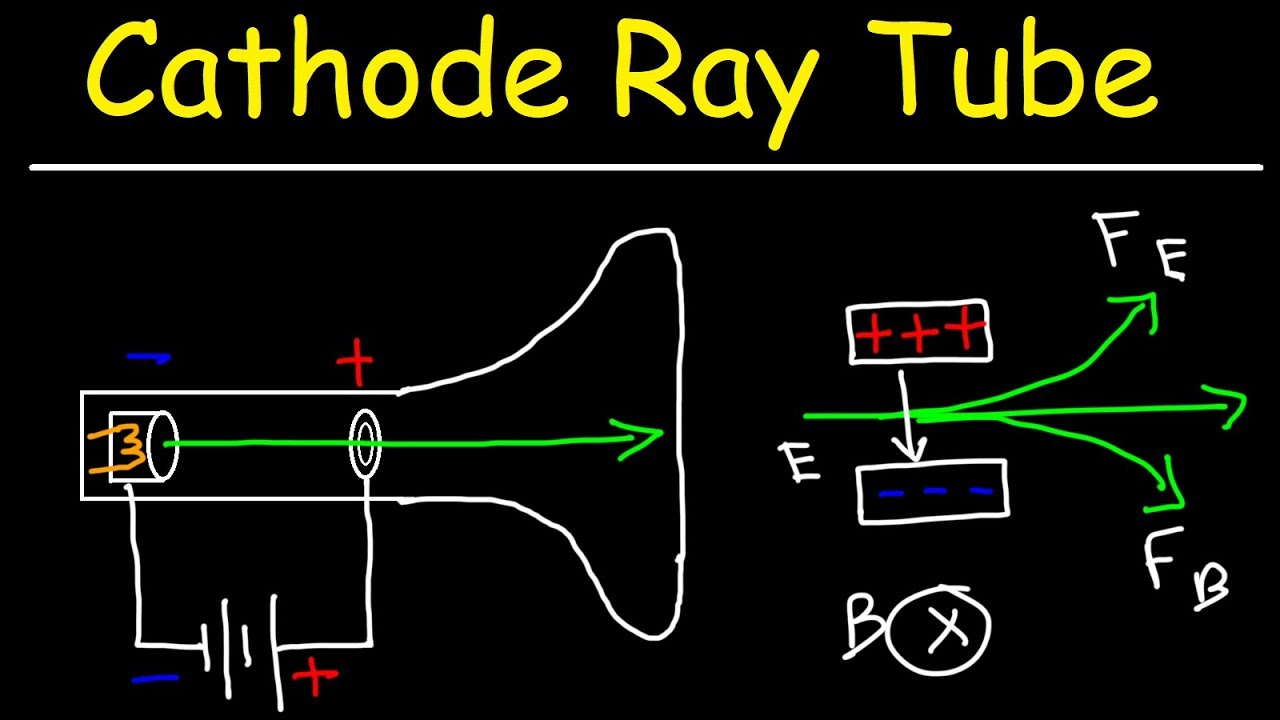
Cathode Ray Tube Experiment and Charge To Mass Ratio of an Electron YouTube
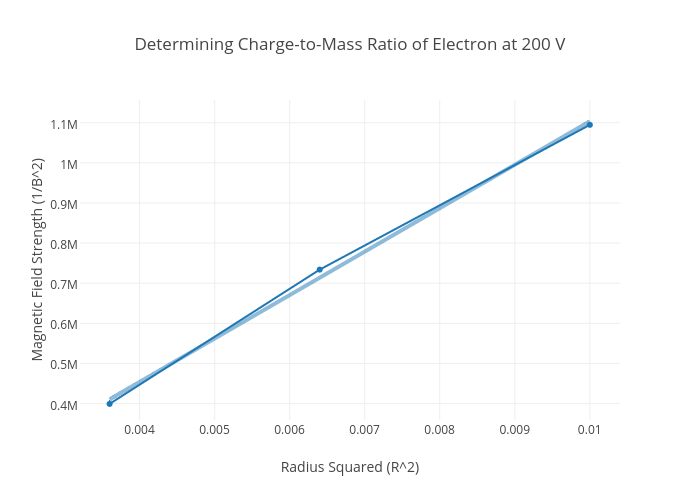
Determining ChargetoMass Ratio of Electron at 200 V scatter chart made by Smagana plotly

Charge to mass ratio of electron proof (equation derivation used in j j thomson experiment
Atomic Structure and Electron Configuration (AQA) — the science hive

PPT Experimental Measurement of the Charge to Mass Ratio of the Electron PowerPoint
Measurement of Charge to Mass ratio of Electron Chemistry Skills

Charge to mass ratio of electron Structure of Atom CHEMISTRY TEACH NCERT Chemistry Class

14Motion of a charged particle in the field

Charge and Mass Ratio of electron part 2 chapter 2 Atoms and their structure YouTube
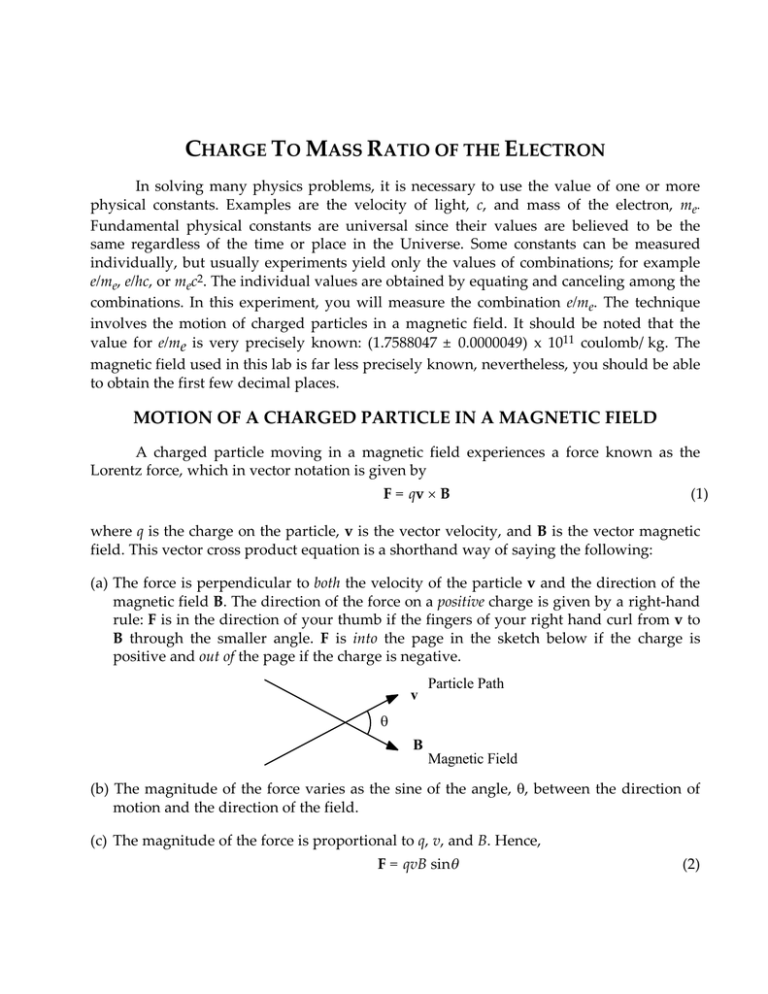
charge to mass ratio of the electron motion of a charged particle in a
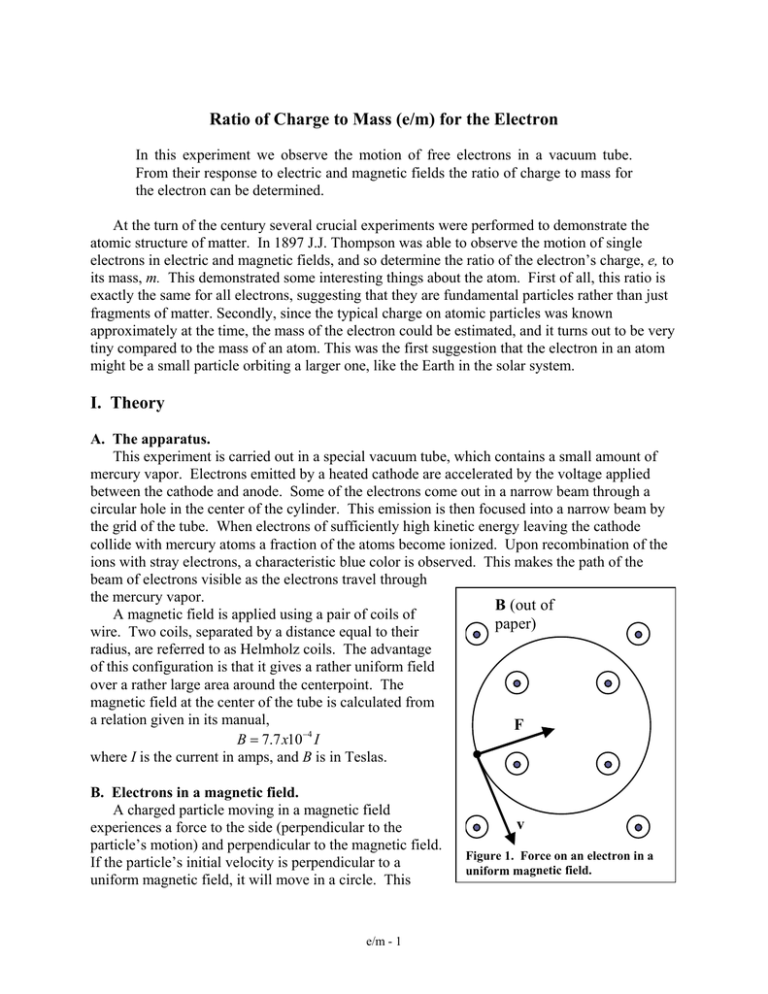
Ratio of Charge to Mass (e/m) for the Electron I. Theory

04 Charge to mass ratio of electron YouTube
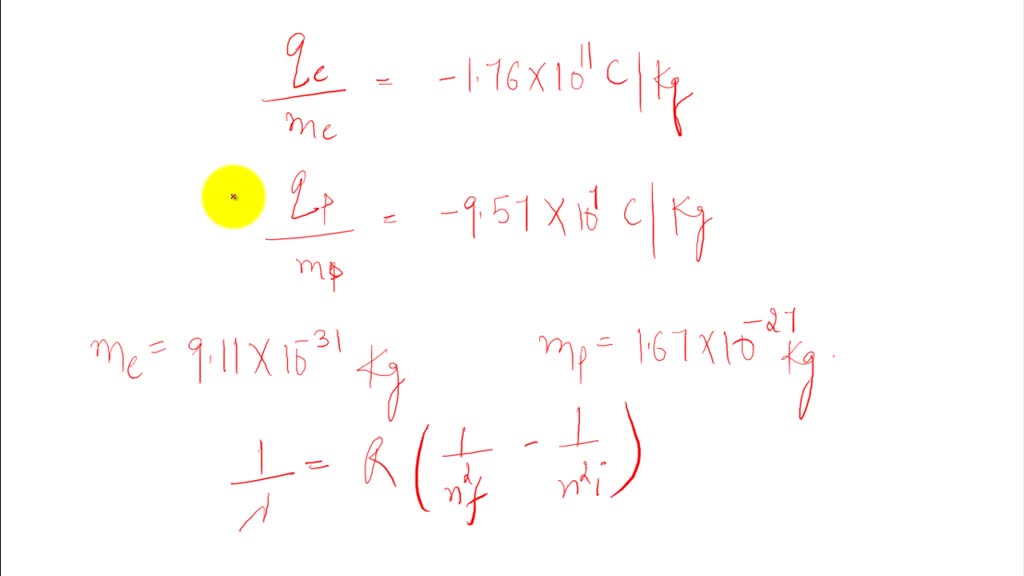
⏩SOLVEDUsing the given chargetomass ratios for electrons and… Numerade

Structure of Atom Class 11 Chemistry Charge to Mass Ratio of Electrons YouTube

Charge to Mass Ratio Mass of Electron Charge of Electron Structure of Atom Part 2
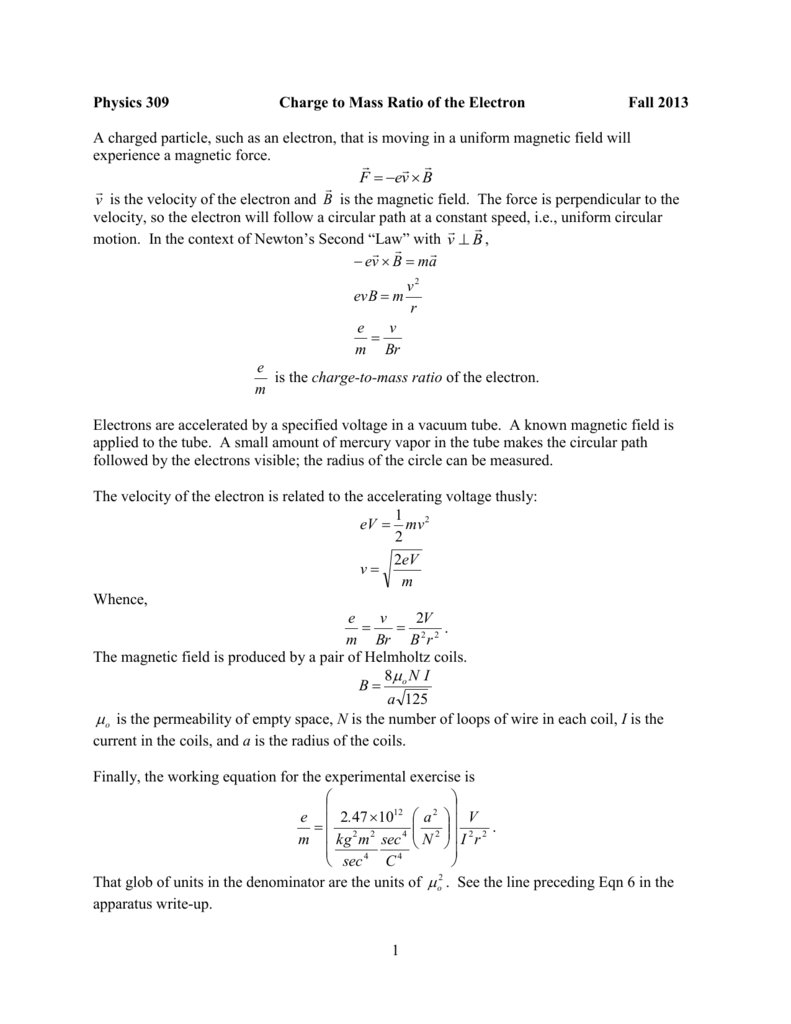
ChargeMass Ratio of the Electron
fields on the electron trajectory (for example, bring a cell phone near the glass bulb). Is it significant enough to affect the measurements? 5. Obtain the value of the charge-to-mass ration, e/m, using the value Be estimated above and relationships described in Equations 9 and 10. References D. J. Griffiths. Magnetostatics, chapter 5, pages.. Thomson determined the ratio of the charge of the electron to its mass, the quantity e/m. ø L v E Figure 2: An electron passes through a region in which there is an electric field E pointing up. The electron is deflected downward by a distance d. Suppose that an electron with charge”-e” and mass m is moving to the right, as shown in Figure 2. It

